Liminatus Pharma Announces New Clinical Milestone in Pancreatic Cancer Screening with Samda Biolab and INNOCS AI
Liminatus Pharma, Inc (NASDAQ:LIMN)
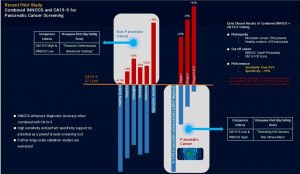
LA PALMA, CA, UNITED STATES, July 7, 2025 /EINPresswire.com/ -- Liminatus Pharma (Nasdaq: LIMN) has announced promising new clinical results that could significantly advance early detection of pancreatic cancer. This development follows the company’s strategic collaboration with Samda Biolab Co., Ltd. and INNOCS AI to establish a research consortium focused on creating a highly accurate, blood-based cancer screening tool.The test is built on the INNOCS AI platform, which combines a proprietary panel of 16 metabolomic biomarkers with artificial intelligence. When used in conjunction with CA19-9—a conventional pancreatic cancer marker—the test achieves remarkably high diagnostic accuracy.
In a recent clinical study involving 1,086 participants, the combined test demonstrated sensitivity between 90% and 98%, with specificity exceeding 99%. Importantly, it successfully identified pancreatic cancers that are often missed by CA19-9 alone, including early-stage and CA19-9-negative cases.
“This could be a game-changer for cancer diagnostics,” said Chris Kim, CEO of Liminatus Pharma. “The test requires just a small blood sample and continues to improve as more data is collected, thanks to the power of AI.”
One of the most promising aspects of this platform is its potential to distinguish early-stage pancreatic cancer from metabolically similar high-risk conditions—such as intraductal papillary mucinous neoplasms (IPMNs), chronic pancreatitis, and other pancreatic cystic lesions—which have historically been difficult to differentiate with conventional methods. The ability to make this distinction using a single screening test from a small blood volume represents a major advance in clinical utility.
The table below highlights the superior sensitivity and specificity of the new test compared to CA19-9 alone, demonstrating its potential to detect cancer both earlier and more reliably.
This new test is non-invasive, cost-effective, and easy to administer, making it an ideal candidate for widespread clinical implementation. Liminatus is currently preparing to expand its trials, enrolling an additional 700 cancer patients and 1,000 healthy individuals to further validate its efficacy.
Depending on the outcome of ongoing studies, the company is evaluating commercialization options, including licensing, strategic partnerships, and acquisitions. Liminatus will also spearhead regulatory submissions in both the United States and South Korea.
“By combining our regulatory expertise with Samda’s biomarker innovations and INNOCS AI’s advanced analytics, we are forming a powerful alliance that is making tangible progress in the fight against cancer,” Kim added.
With the global market for pancreatic cancer diagnostics projected to surpass $3 billion by 2026, this collaboration positions Liminatus Pharma at the forefront of next-generation cancer detection.
About Liminatus Pharma
Liminatus Pharma (Nasdaq: LIMN) is a preclinical-stage immuno-oncology company advancing IBA101 toward best-in-human trials. Building on over a decade of CD47 research and lessons learned from industry setbacks, Liminatus’s mission is to develop next-generation immunotherapies that restore immune balance—bridging innate and adaptive immunity to drive safer, more durable anti-tumor responses.
Forward-Looking Statements
Cautionary Note Regarding Forward-Looking Statements Statements in this press release may constitute "forward-looking statements" under the Private Securities Litigation Reform Act of 1995. All statements, other than statements of present or historical fact, that address activities, events or developments that Liminatus Pharma, Inc. expects, believes or anticipates will or may occur in the future, including statements about results of operations and financial condition, expected future results, expected benefits from our investment and strategic plans and other initiatives, and expected future growth and profitability, are forward-looking statements. The words "will," "may," "believes," "anticipates," "thinks," "expects," "estimates," "plans," "intends" and similar expressions are intended to identify forward-looking statements. Forward-looking statements involve risks and uncertainties that could cause actual results to differ materially from those anticipated by these forward-looking statements. In addition, statements which refer to expectations, projections or other characterizations of future events or circumstances, statements involving a discussion of strategy, plans or intentions, statements about management's assumptions, projections or predictions of future events or market outlook and any other statement other than a statement of present or historical fact are forward-looking statements.
For more information, please contact:
Chris Kim, Chief Executive Officer chris@liminatus.com 213-273-5453 www.liminatuspharma.com
Chris Kim
Liminatus Pharma, Inc
+1 213-273-5453
email us here
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.